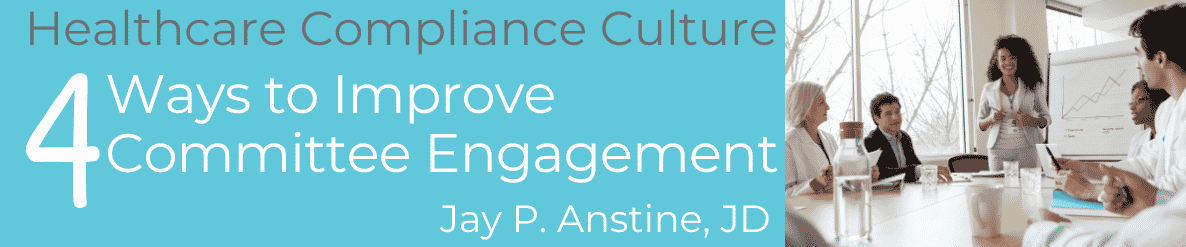Be a partner and resource. Four C’s of effective healthcare compliance: Communication, Collaboration, Credibility, and Culture – Brian Kozik
Continue readingA culture of compliance through proactive decision-making
Does your healthcare organization make proactive decisions about regulatory changes with time to adjust? Three steps to help make decisions farther upstream.
Continue readingTwo metrics that demonstrate the impact of your compliance culture
Metrics for healthcare compliance culture impact. OIG Supplemental Compliance Program Guidance 2005, and the Evaluation of Corporate Compliance Programs, 2020.
Continue readingTwo tips for enabling a speak-up culture in your healthcare organization
A speak-up culture is earned. Invest time with colleagues across your healthcare organization. Develop open lines of communication for effective compliance.
Continue readingCompliance Committee Engagement Strategies That Work
Healthcare compliance committees offer the operational impact of compliance activities and drive culture. Four strategies to improve engagement-Jay Anstine
Continue readingTrust in healthcare compliance programs
Four ways to build a healthy culture of compliance. Help your clinical and operational colleagues see compliance as foundational to your healthcare organization
Continue readingHealthcare compliance culture means doing the right thing at all levels
Healthcare compliance culture is strategic. Defining compliance culture in the context of overall healthcare priorities. Measure it.
Continue readingFour Power Skills for Influence
Compliance helps healthcare organizations mitigate risk by building strong relationships and leading through influence for effective regulatory change management
Continue reading12 key metrics for compliance officers looking to move their culture forward
Culturally impactful metrics emphasize getting healthcare compliance officers in the right rooms – ahead of regulatory change or organizational growth.
Continue reading